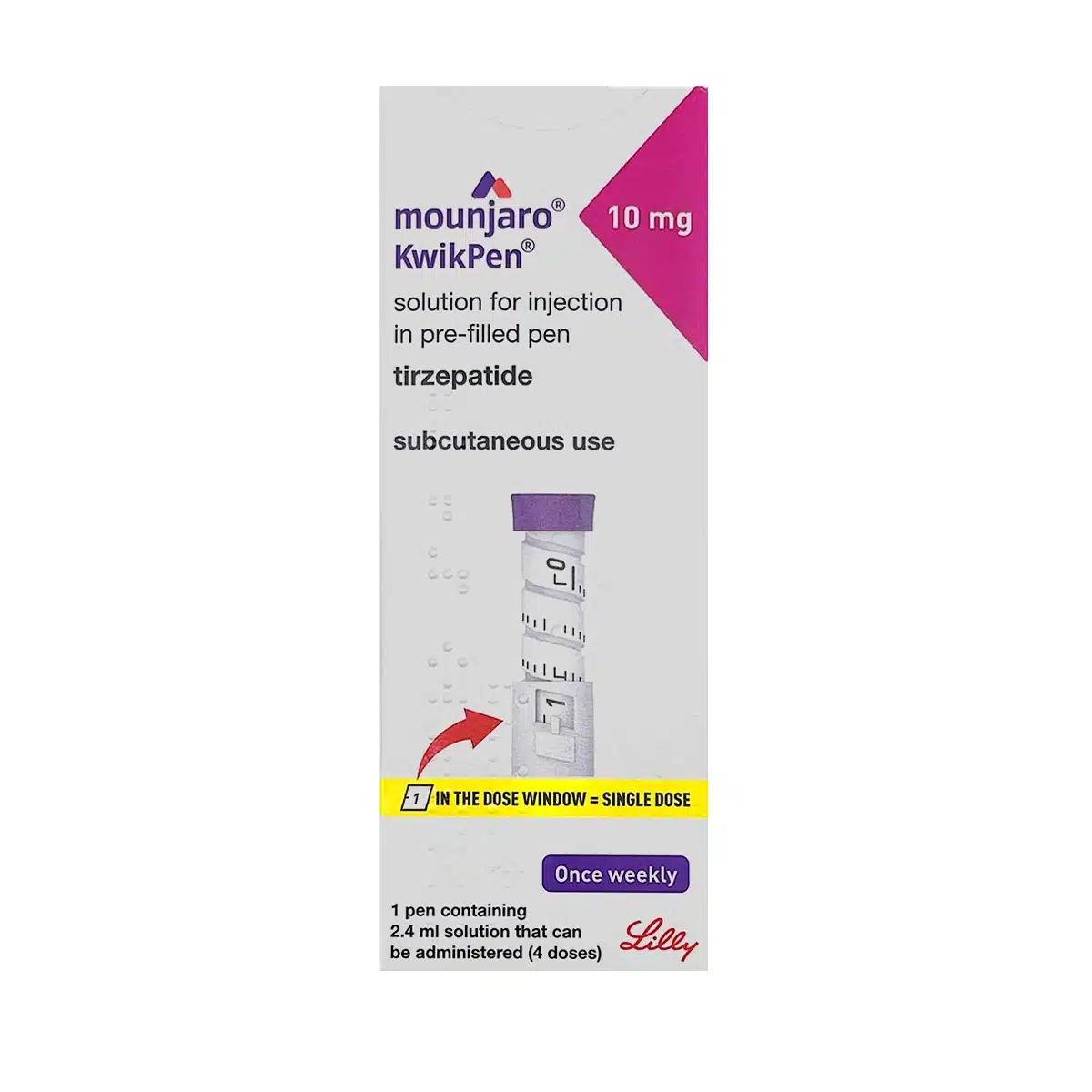
What is MOUNJARO® 10mg KwikPen®
The MOUNJARO® 10mg KwikPen® is a multi-dose, disposable pen pre-filled with solution delivering 10 mg tirzepatide in 0.6 mL once per week. It is prescribed for adult patients with type 2 diabetes mellitus and for long-term weight management in specific BMI-defined populations. Each pen contains 2.4 mL solution (40 mg total), providing four weekly injections. The formulation is clear, colourless to slightly yellow and is intended for subcutaneous use in the abdomen, thigh, or upper arm.
- Per dose: 10 mg tirzepatide in 0.6 mL
- Total pen content: 40 mg/2.4 mL (16.7 mg/mL)
- Doses per pen: 4
- Administration route: Subcutaneous
- Injection sites: Abdomen, thigh, upper arm
What is the Administration Protocol for MOUNJARO® 10mg KwikPen®
The 10 mg strength is a common maintenance dose. It is delivered on the same day each week, with the option to shift injection day if dosing intervals are ≥3 days.
- Initiation: Begin with 2.5 mg once weekly for 4 weeks, then titrate upward
- Escalation: Advance in increments of 2.5 mg at ≥4 week intervals until reaching 10 mg
- Maintenance: 5 mg, 10 mg, or 15 mg weekly depending on patient needs
- Missed dose:
- If ≤4 days late: inject promptly
- If >4 days late: skip dose and continue with regular schedule
- Administration steps:
- Always attach a new sterile needle
- Dial to 1 in the window (=0.6 mL dose)
- Inject subcutaneously, hold for 5 seconds until window reads 0
- Rotate injection sites regularly to prevent irritation
What are the Indications for MOUNJARO® 10mg KwikPen®
This pen strength is indicated for both glycaemic control and weight regulation in adults. Clinical restrictions and contraindications apply.
- Indications:
- Type 2 diabetes mellitus, as monotherapy or with other agents
- BMI ≥30 kg/m², or BMI ≥27 to <30 kg/m² with related comorbidities (e.g., hypertension, OSA, dyslipidaemia, cardiovascular disease, prediabetes)
- Contraindications:
- Hypersensitivity to tirzepatide or excipients
- Precautions:
- Severe gastrointestinal disease (gastroparesis)
- History of pancreatitis
- Adverse effects:
- Frequent: nausea, diarrhoea, constipation, vomiting, abdominal pain
- Hypoglycaemia, especially with insulin or sulphonylureas
- Localised injection reactions (approx. 5%)
- Rare: pancreatitis, hypersensitivity
What is the composition of MOUNJARO® 10mg KwikPen®
The solution contains tirzepatide and carefully selected excipients.
- Active substance: Tirzepatide 10 mg/0.6 mL
- Total pen content: 40 mg/2.4 mL
- Concentration: 16.7 mg/mL
- Excipients: Benzyl alcohol (~5.4 mg/dose), disodium hydrogen phosphate heptahydrate, glycerol, phenol, sodium chloride, sodium hydroxide, hydrochloric acid, water for injections
- Sodium level: <1 mmol sodium per dose
- Appearance: Clear, colourless to slightly yellow solution
What Comes in the MOUNJARO® 10mg KwikPen® Package
The packaging includes the pen and essential instructions, but needles are not supplied.
- Device presentation: KwikPen® with 2.4 mL solution (4 doses)
- Pack sizes: 1 or 3 pens
- Needles: Must be obtained separately
- Documentation: Instructions for Use and Patient Information Leaflet
Does MOUNJARO® 10mg KwikPen® cause side effects?
Adverse reactions are most often gastrointestinal and occur most frequently during escalation.
- Very common: Nausea, diarrhoea, vomiting, abdominal pain, constipation
- Hypoglycaemia risk increased with sulphonylureas or insulin
- Injection-site reactions: Mild, ~5% of cases
- Gallbladder events: Occasionally reported
- Rare but serious: Acute pancreatitis, severe hypersensitivity
- Laboratory findings: Elevated amylase and lipase
How should MOUNJARO® 10mg KwikPen® be stored?
Pens must be kept under controlled conditions.
- Unopened pens: Store at 2–8 °C
- Do not freeze; discard if frozen
- In-use pens: Store at ≤30 °C for up to 30 days
- Discard after 30 days, regardless of remaining liquid
- Needles: Remove after injection, discard in sharps container
- Keep out of reach of children