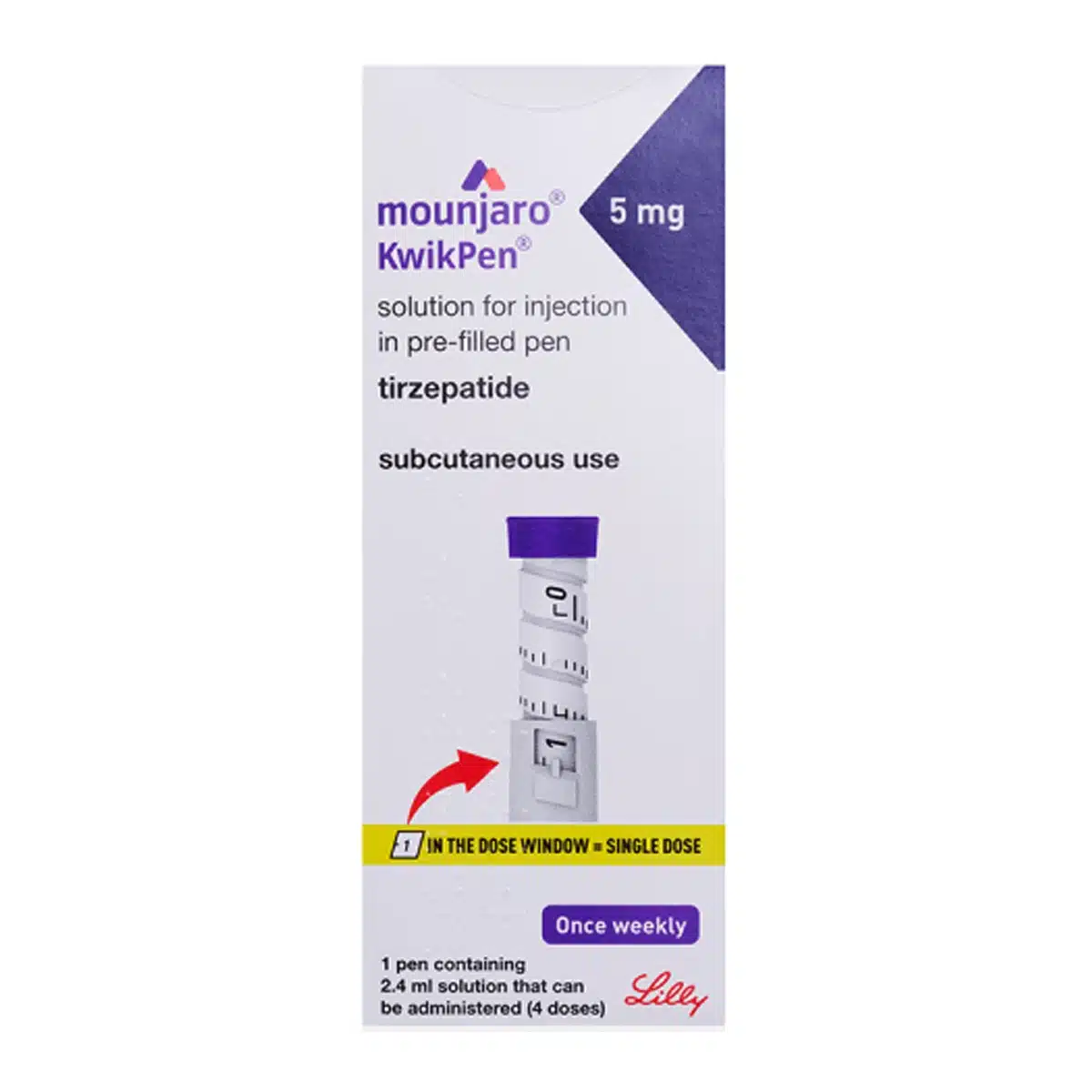
What is MOUNJARO® 5mg KwikPen®
The MOUNJARO® 5mg KwikPen® is a pre-filled, disposable injection device designed to deliver a precise 5 mg dose of tirzepatide in 0.6 mL once weekly. It is indicated for the management of type 2 diabetes in adults and for weight reduction in eligible patients. Each pen contains 2.4 mL of solution with a total of 20 mg tirzepatide, providing four fixed doses. The liquid preparation is clear, colourless to slightly yellow and is injected subcutaneously into the abdomen, thigh, or upper arm.
- Strength per injection: 5 mg tirzepatide (0.6 mL)
- Total content: 20 mg/2.4 mL solution (8.33 mg/mL)
- Doses per pen: 4
- Administration route: Subcutaneous
- Injection areas: Abdomen, thigh, upper arm
What is the Administration Protocol for MOUNJARO® 5mg KwikPen®
The 5 mg pen is typically used as the first maintenance dose following initiation at 2.5 mg. It is administered once every seven days at a consistent time.
- Initiation regimen: Start with 2.5 mg once weekly for 4 weeks, then increase to 5 mg
- Titration: Further increments of 2.5 mg every ≥4 weeks may be used up to a maximum of 15 mg
- Maintenance options: 5 mg, 10 mg, or 15 mg weekly
- Missed dose protocol:
- If ≤4 days late: inject as soon as possible
- If >4 days late: omit and continue with next scheduled dose
- Administration technique:
- Attach new needle each time
- Dial pen until 1 shows in the dose window (=0.6 mL)
- Inject and hold for 5 seconds until window shows 0
- Rotate sites weekly to reduce local irritation
What are the Indications for MOUNJARO® 5mg KwikPen®
The product is approved for both glycaemic control in type 2 diabetes and weight management in adults with obesity or overweight and related comorbidities.
- Indications:
- Monotherapy or combination therapy for type 2 diabetes
- Adults with BMI ≥30 kg/m²
- Adults with BMI ≥27 to <30 kg/m² and comorbidities such as hypertension, dyslipidaemia, OSA, or cardiovascular disease
- Contraindications:
- Hypersensitivity to tirzepatide or excipients
- Precautions:
- History of pancreatitis
- Severe gastrointestinal disease (e.g., gastroparesis)
- Adverse effects:
- Common: nausea, diarrhoea, vomiting, constipation, abdominal pain
- Risk of hypoglycaemia when combined with sulphonylurea or insulin
- Local injection-site reactions possible
- Rare: acute pancreatitis, hypersensitivity
What is the composition of MOUNJARO® 5mg KwikPen®
The formulation contains tirzepatide as the active substance, together with stabilizing excipients.
- Active ingredient: Tirzepatide 5 mg/0.6 mL
- Total content: 20 mg/2.4 mL
- Concentration: 8.33 mg/mL
- Excipients: Benzyl alcohol (~5.4 mg/dose), disodium hydrogen phosphate heptahydrate, glycerol, phenol, sodium chloride, sodium hydroxide, hydrochloric acid, water for injections
- Sodium level: <1 mmol sodium per dose
- Appearance: Clear, colourless to slightly yellow solution
What Comes in the MOUNJARO® 5mg KwikPen® Package
Each package contains the injection device and documentation.
- Device: 2.4 mL KwikPen® with 4 doses
- Pack sizes: 1 or 3 pens
- Needles: Not supplied with pack
- Documentation: Instructions for Use and Patient Leaflet
Does MOUNJARO® 5mg KwikPen® cause side effects?
Adverse reactions are possible, mainly gastrointestinal, particularly during initiation.
- Very common: Nausea, vomiting, diarrhoea, constipation, abdominal pain
- Hypoglycaemia: Increased risk with insulin or sulphonylureas; severe events rare (~0.2%)
- Injection-site reactions: Reported in 3–8% of patients
- Gallbladder events: Seen in up to 2%
- Rare: Pancreatitis, hypersensitivity reactions
- Lab findings: Increases in amylase and lipase
How should MOUNJARO® 5mg KwikPen® be stored?
Strict storage is required to preserve integrity.
- Unopened pens: Refrigerate at 2–8 °C
- Do not freeze; discard if frozen
- After first use: Store at ≤30 °C for 30 days maximum
- Discard after 30 days regardless of remaining content
- Needles: Remove after use, discard safely
- Keep out of reach of children