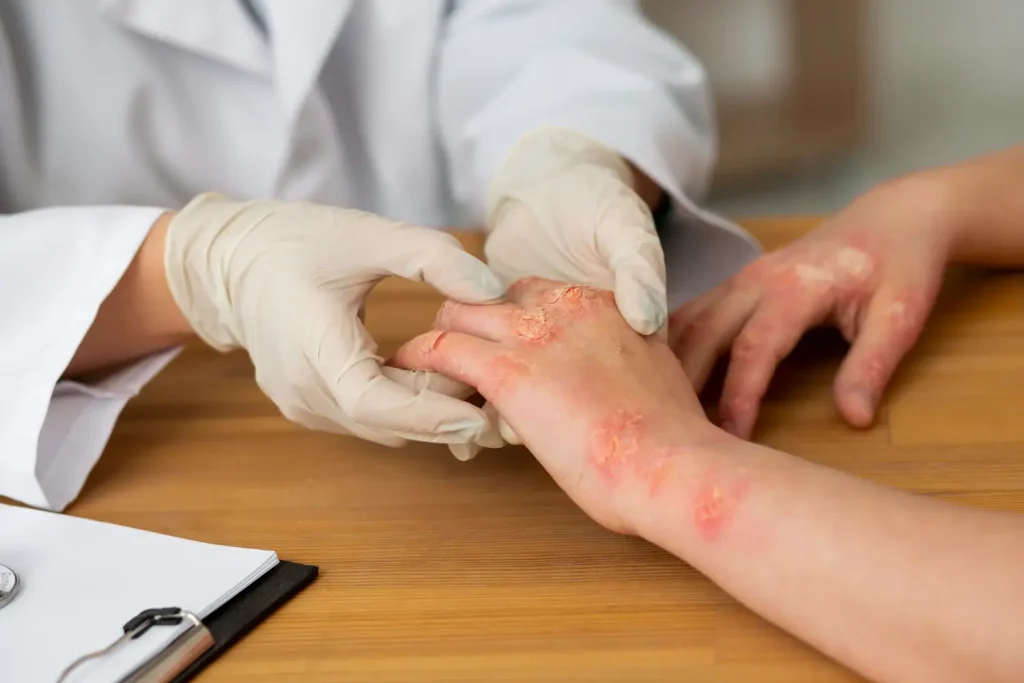
Psoriasis is a chronic inflammatory condition that affects more than 100 million people worldwide. Its hallmark is the appearance of raised, scaly skin plaques, but the impact goes beyond the surface. For many, psoriasis affects daily comfort, self-confidence, and can be linked with other conditions such as psoriatic arthritis, cardiovascular disease, and depression.
For adults living with moderate-to-severe plaque psoriasis, treatment often requires more than topical creams. When systemic therapy or phototherapy is needed, Cimzia (certolizumab pegol) is a TNF-α inhibitor approved by the FDA for this use. By reducing inflammation, Cimzia can help clear the skin and improve quality of life. In clinical studies, more than half of patients reached clear or nearly clear skin within 16 weeks, and many were able to maintain those improvements over time.
In this article, we’ll take a closer look at how Cimzia works for plaque psoriasis, who may be a good candidate for treatment, and what patients should discuss with their healthcare providers before starting therapy.
Key Takeaways
- Cimzia (certolizumab pegol) is a biologic TNF-alpha inhibitor used to treat moderate to severe plaque psoriasis.
- Clinical trials have shown that many patients achieve clear or nearly clear skin within 16 weeks, with sustained results lasting up to 96 weeks.
- Difficult-to-treat areas, such as scalp and nail psoriasis, also respond to Cimzia, improving both symptoms and quality of life.
- Safety monitoring is crucial: risks include infections (TB, opportunistic), malignancy, neurologic disorders, and worsening heart failure. Pre-treatment TB screening, lab tests, and vaccine checks are recommended.
- Common side effects include mild infections, headaches, and injection site reactions, which are usually manageable.
- Cimzia offers unique reassurance for women of childbearing potential due to minimal placental and breast milk transfer, making it a viable option in pregnancy or postpartum when needed.
- The drug’s PEGylated structure extends its half-life, allowing for flexible dosing schedules (every 2 or 4 weeks).
- Successful psoriasis management with Cimzia depends on shared decision-making, regular monitoring, and consistent follow-up with healthcare providers.
About: Medical Spa RX provides medical practices with premium products at the best prices. If you’re looking to buy Cimzia online for your practice, the sales representatives at Medical Spa RX can give you guidance.
Mechanism of Action: How Cimzia Targets TNF-Alpha in Psoriasis
Cimzia (certolizumab pegol) belongs to a group of medicines known as TNF inhibitors, which work by blocking tumor necrosis factor-alpha (TNF-α)—a protein that fuels inflammation in autoimmune diseases. Patients often ask, “Is Cimzia a biologic?” The answer is yes. Cimzia is a biologic therapy designed to regulate immune activity more precisely than traditional drugs.
In plaque psoriasis, TNF-α stimulates the rapid overproduction of skin cells, resulting in redness, thickened plaques, and scaling. Cimzia binds directly to TNF-α in both the bloodstream and inflamed tissues, blocking its effects and calming this overactive process. Unlike biologics that target interleukins, Cimzia focuses solely on TNF-α, producing measurable improvements in psoriatic plaques.
Its PEGylated Fab’ fragment design extends its half-life, meaning the drug stays active in the body for longer periods. This allows for more flexible dosing without sacrificing effectiveness. By disrupting one of the primary inflammatory pathways in psoriasis, Cimzia offers patients clearer skin and sustained symptom relief, making it a reliable option for long-term management.
Clinical Evidence: Cimzia Efficacy in Moderate-to-Severe Plaque Psoriasis
Cimzia’s effectiveness has been well-documented in large clinical trials involving patients with moderate-to-severe plaque psoriasis. One of the primary measures used in these studies is the Psoriasis Area and Severity Index (PASI), which tracks improvements in skin clearance.
Within the first 16 weeks of treatment, a significant number of patients achieved PASI 75 and PASI 90 responses, meaning their symptoms improved by 75% to 90%. For many, visible skin clearance occurred rapidly during the induction phase.
Long-term studies have shown that these benefits are not short-lived. Patients maintained strong PASI responses for up to 48 to 96 weeks, reflecting durable control of psoriasis symptoms. In addition, Cimzia has shown effectiveness in difficult-to-treat areas, including scalp and nail psoriasis, where symptoms can be especially bothersome. Patients also reported improvements in quality of life, with less itching, scaling, and discomfort interfering with daily activities.
Together, these findings position Cimzia as a dependable treatment for patients needing sustained relief and durable disease control.
Safety Considerations and Patient Monitoring in Cimzia Psoriasis Therapy
Like other TNF inhibitors, Cimzia has a safety profile that requires attention from both patients and healthcare providers. Most people tolerate treatment well, but proactive monitoring helps reduce risks.

Key safety considerations include:
- Common Side Effects: Mild issues like upper respiratory infections, headaches, and injection site reactions may occur. These are usually manageable.
- Infection Risks: Cimzia can increase susceptibility to serious infections, including tuberculosis and opportunistic pathogens. Screening and monitoring are essential.
- Pre-Treatment TB Screening: All patients should undergo testing for latent tuberculosis before starting therapy, with preventive treatment provided if needed.
- Malignancy and Neurologic Monitoring: Rare risks include lymphoma, other malignancies, and demyelinating disorders, requiring careful patient evaluation.
- Cardiac Safety: Cimzia may worsen heart failure, so monitoring for cardiac symptoms is essential, especially in at-risk individuals.
- Laboratory Tests: Periodic blood counts and liver function tests help detect abnormalities early.
- Vaccination Status: Live vaccines are contraindicated; immunizations should be updated before treatment initiation.
- Patient Education: Patients should be informed about the signs of infection and encouraged to attend regular follow-up appointments.
Special Populations: Women of Childbearing Potential and Cimzia Use
Cimzia offers a unique advantage for women of childbearing potential because of its limited placental transfer compared to other biologics. This is due to Cimzia’s structure—it lacks the Fc region that normally facilitates crossing into fetal circulation.
Data from clinical studies and pregnancy registries suggest Cimzia can be used when treatment is necessary during pregnancy, providing disease control with reduced fetal exposure. For women planning pregnancy or already expecting, Cimzia may therefore be a viable option under medical guidance.
Breastfeeding research, including data from the CRADLE study, also shows minimal transfer of Cimzia into breast milk, adding reassurance for mothers who require treatment postpartum.
Still, decisions should always be individualized. Physicians weigh the benefits of disease control against any potential risks, taking into account alternative options. Shared decision-making and pre-treatment counseling remain essential. Cimzia’s reassuring safety profile in this population makes it an important consideration for women managing psoriasis during their reproductive years.
Conclusion
Cimzia is a proven biologic therapy for adults with moderate-to-severe plaque psoriasis. By directly targeting TNF-α, it offers sustained improvements in skin clearance, with clinical studies confirming both short-term effectiveness and long-term durability. Its design allows for flexible dosing, and its unique safety profile makes it especially relevant for women of childbearing potential.
When prescribing Cimzia, healthcare providers balance patient history, disease severity, and safety requirements with lifestyle considerations. For patients, this means committing to regular injections, ongoing monitoring, and active participation in care. While no single therapy is perfect for everyone, Cimzia stands out as a valuable, reliable option in the evolving management of psoriasis.
FAQs
1. What is Cimzia, and how does it help in psoriasis?
Cimzia is a TNF inhibitor that blocks TNF-α, reducing inflammation and slowing the overproduction of skin cells that cause psoriatic plaques.
2. How effective is Cimzia for plaque psoriasis?
Clinical trials show strong results, with many patients experiencing significant plaque clearance within 16 weeks and sustained improvement with continued treatment.
3. What is the recommended dosing for Cimzia in psoriasis?
Treatment usually begins with induction doses, followed by maintenance injections every two or four weeks depending on response.
4. What are the safety concerns with Cimzia therapy?
Risks include infections, TB reactivation, and rare complications like malignancies. Careful monitoring and screening reduce these risks.
5. Can women use Cimzia during pregnancy?
Yes. Cimzia has minimal placental transfer and may be considered when treatment is necessary. Decisions should be guided by a specialist.
References
World Health Organization. Global report on psoriasis. 2016. Accessed September 23, 2025. https://iris.who.int/bitstream/handle/10665/204417/9789241565189_eng.pdf.psoriasis?sequence=1
Augustin M, Gottlieb AB, Lebwohl M, et al. Complete Skin Clearance is Associated with the Greatest Benefits to Health-Related Quality of Life and Perceived Symptoms for Patients with Psoriasis. Dermatology and Therapy. 2024;14(10):2841-2857. doi:10.1007/s13555-024-01261-6
Mease P, Deodhar A, Fleischmann R, et al. Effect of certolizumab pegol over 96 weeks in patients with psoriatic arthritis with and without prior antitumour necrosis factor exposure. RMD Open. 2015;1(1):e000119. doi:10.1136/rmdopen-2015-000119
Mariette X, Förger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77(2):228-233. doi:10.1136/annrheumdis-2017-212196