South Korea’s botulinum toxin type A market hit a value of USD 194.1 million in 2023 and is expected to grow at a healthy CAGR of 10.4% through 2030. An increasing demand for aesthetic procedures and ongoing innovations from leading domestic brands essentially drives this growth. Prominent South Korean manufacturers like Hugel and Huons Biopharma are shaking up the global scene by offering cost-effective alternatives to established products, all while maintaining comparable clinical performance.
One of these innovations is Liztox, a botulinum toxin formulation developed by Huons Biopharma. Known for its ability to deliver subtle yet noticeable results while maintaining natural facial expressiveness, Liztox is becoming a favorite among patients and practitioners alike. Many patients appreciate how it enhances their features without the “frozen” look, and practitioners often note its smooth injection feel, consistency, and ease of use.
This article will take a closer look at Liztox from both the patient and practitioner perspectives, offering insights into the reported outcomes, treatment experiences, and overall satisfaction with this emerging aesthetic option.
Key Takeaways
- Liztox (HU-014) is a botulinum toxin type A product that has approval from the Korean MFDS/KFDA. It is available in several global markets, but it is not yet FDA-approved for use in the U.S.
- Clinicians praise Liztox for its smooth reconstitution, consistent injection flow, and ease of use, making it a reliable choice for both therapeutic and cosmetic applications.
- Patients report natural-looking results with Liztox, including smoother forehead lines, softened frown lines, and reduced crow’s feet, all while maintaining facial expressiveness.
- Liztox has a rapid onset with visible effects typically appearing within 3–5 days, and peak results are reached within 1–2 weeks. It offers a duration of effect of 12 weeks in therapeutic use, while aesthetic results can last up to 4–5 months in some patients.
- Liztox is known for its precise targeting and minimal diffusion, which helps avoid the spread to adjacent muscles, contributing to predictable outcomes in dynamic facial areas.
- The most common side effects are temporary redness, swelling, bruising, and headache, all of which resolve within a few days. Serious side effects are rare but emphasize the need for skilled administration.
About: Medical Spa RX provides medical practices with premium products at the best prices. If you’re looking to buy Liztox online for your practice, the sales representatives at Medical Spa RX can give you guidance.
Practitioner Feedback on Handling and Injection Experience
Liztox (HU-014) has garnered positive feedback from clinicians for its handling and injection characteristics. In a multicenter, double-blind Phase 3 trial comparing Liztox to Botox for the treatment of post-stroke upper limb spasticity, researchers noted that Liztox reconstituted smoothly, with minimal foaming. This allowed for accurate dosing and consistent injection flow, which are critical for achieving reliable outcomes in both therapeutic and aesthetic treatments.

The recommended dilution of 2.5 mL of saline per 100 units has been praised for supporting precise targeting and predictable diffusion, particularly in smaller muscle groups. This is important for practitioners who need to maintain control during injections and avoid any undesirable spread to adjacent areas.
During the administration process, clinicians reported no clogging or viscosity issues, making the injection experience smoother and more efficient. Patient comfort scores were also comparable to Botox, further supporting Liztox’s viability as an alternative botulinum toxin for both therapeutic and cosmetic applications.
Patient-Reported Outcomes on Efficacy and Natural Results
Now available under the brand name Hutox, Liztox continues to receive high praise from patients for providing natural-looking results without compromising facial expressiveness. Patients frequently report noticeable improvements, such as smoother forehead lines, softened frown lines or wrinkles, and a reduction in crow’s feet, while still retaining their ability to show dynamic facial expressions.
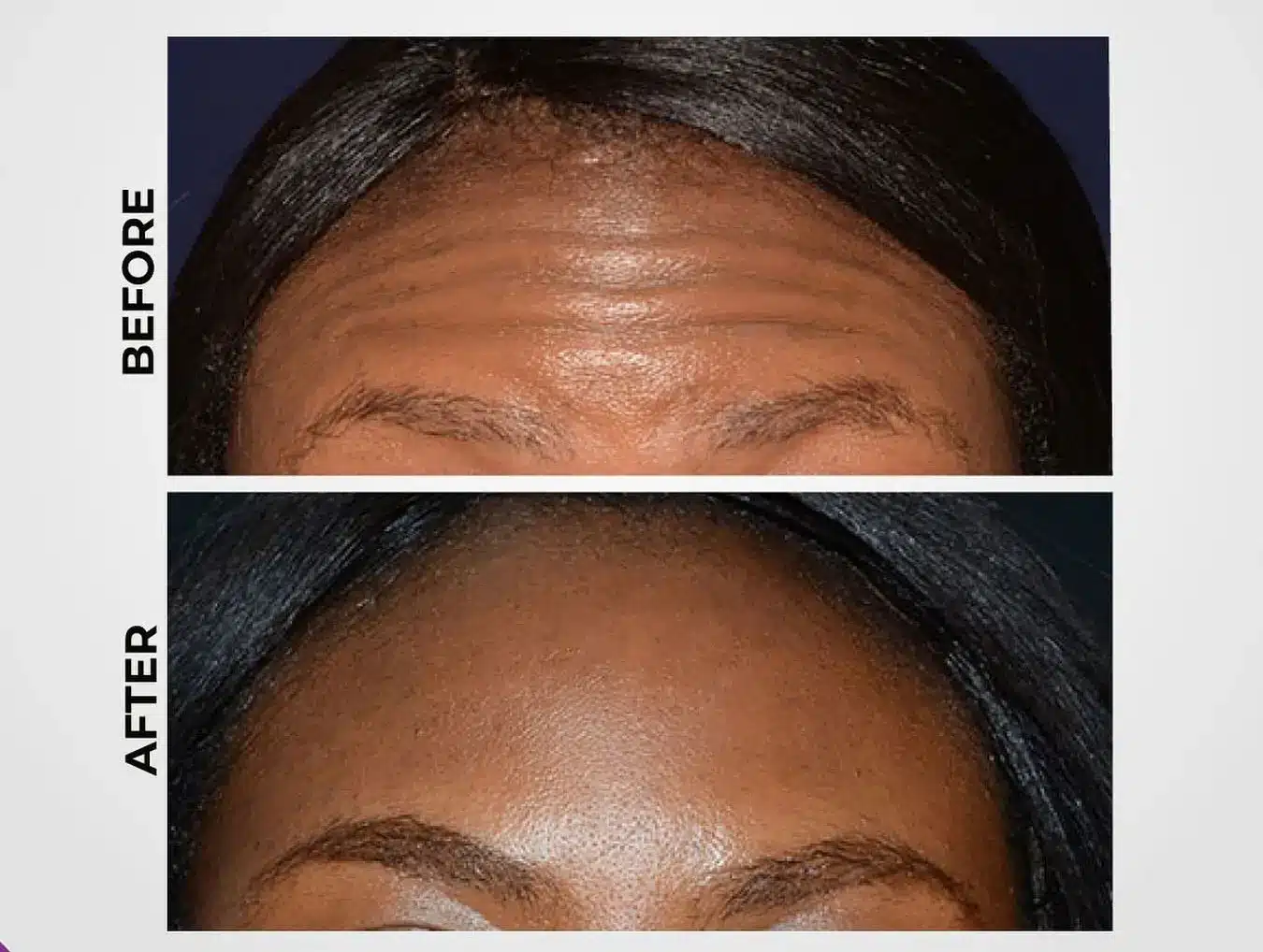
This subtle, understated enhancement has become a preferred choice for those seeking a more natural rejuvenation rather than a heavily “frozen” appearance.

Many patients also note improvements in skin texture, resulting in a more refreshed appearance overall. This aligns with the modern aesthetic trend that favors more personalized treatments with minimal downtime.
One frequent question from patients is, “Is Liztox FDA approved?” While Liztox has not yet received FDA approval in the United States, it has been approved by the Korean MFDS/KFDA and is available in other global markets. This gives patients the confidence that the product is regulated and safe for use in countries where it is approved.
Clinicians often highlight Liztox as an effective option for those seeking visible improvements without drastic changes. This aligns with the growing trend toward subtle, personalized aesthetic procedures that cater to individual patient needs.
Longevity, Onset, and Real-World Performance Data
Liztox (HU-014) is known for its rapid onset of action. Most patients begin to see visible effects within 3–5 days after treatment. Peak results typically occur within 1–2 weeks, making it an appealing choice for those looking for timely aesthetic improvements. This fast-acting profile ensures that patients experience quick results, which is often a significant consideration for those looking for a refreshed appearance without waiting too long.
Therapeutic data suggests an average duration of effect around 12 weeks. This figure is consistent with the longevity observed for other botulinum toxin products like Botox and Xeomin. However, many aesthetic patients report that the effects of Liztox can extend to 4–5 months. This extended duration is beneficial for patients who prefer longer-lasting results with fewer touch-ups.
In real-world practice, most patients choose to return for retreatment every 3–4 months, but this varies depending on factors such as muscle activity, individual treatment goals, and desired outcomes. Liztox’s controlled diffusion and minimal spread to adjacent muscles make it particularly effective in dynamic facial zones, such as the glabella, forehead, and crow’s feet.
Commonly Reported Side Effects and Safety Considerations
As with all botulinum toxin treatments, Liztox is generally safe when administered by qualified professionals. The most commonly reported side effects are mild and temporary, typically resolving within a few days. These include:
- Localized Redness or Swelling: A common and short-term reaction. It typically subsides within a few hours after treatment.
- Mild Bruising: This can occur from needle insertion and usually fades within several days without complications.
- Temporary Tenderness or Discomfort: Mild sensitivity at the injection sites, generally subsiding within 24–48 hours.
- Headache: Occasionally reported post-treatment, but typically mild and self-limiting without the need for medication.
- Slight Asymmetry: Minor imbalance that may occur at the start. This often corrects itself naturally as the product settles within 1–2 weeks.
While rare, more serious side effects can occur if the product is not administered correctly. This emphasizes the importance of seeking treatment from trained medical providers who are well-versed in botulinum toxin injections. Patients are typically advised to follow post-treatment guidelines. These include avoiding strenuous exercise and not lying down for several hours after treatment, to minimize risks and optimize results.
Conclusion
Liztox earns strong reviews from both practitioners and patients for its combination of performance, safety, and natural-looking outcomes. Practitioners appreciate its precise handling, making it easier to achieve consistent results. Meanwhile, patients value the subtle yet noticeable improvements it delivers. With its relatively quick onset, predictable duration, and targeted diffusion, Liztox has established itself as a reliable choice for both cosmetic and therapeutic applications.
As with any aesthetic treatment, success is not only about the product itself but also the skill of the injector. Choosing a qualified provider ensures safe administration and results that align with individual patient goals.
FAQs
1. What do doctors use Liztox for?
Liztox temporarily reduces facial lines and wrinkles by relaxing targeted muscles.
2. How soon will I see results after Liztox?
Most patients notice improvements within 3 to 5 days, with full results in about 1 to 2 weeks.
3. How long does Liztox last?
Results typically last around 3 to 4 months, depending on individual factors and treatment areas.
4. Is Liztox safe?
Yes, when administered by qualified professionals, Liztox has a strong safety record with mostly mild, short-term side effects.
5. Can Liztox target concerns other than wrinkles?
Yes, practitioners may use Liztox for other approved medical and aesthetic purposes, depending on patient needs.
6. Are there any restrictions after Liztox treatment?
Experts usually advise patients to avoid strenuous activity, heat exposure, and lying flat for several hours post-treatment.
7. Who should avoid Liztox?
Individuals who are pregnant, breastfeeding, or have certain neuromuscular conditions should not receive Liztox injections.
References
South Korea Botulinum Toxin Type A Market Size Report 2030. https://www.grandviewresearch.com/industry-analysis/south-korea-botulinum-toxin-type-a-market-report
Ye DH, Chun MH, Park YG, et al. A Randomized, Double-Blind, Active Control, Multicenter, Phase 3 Study to Evaluate the Efficacy and Safety of Liztox® versus Botox® in Post-Stroke Upper Limb Spasticity. Toxins. 2023;15(12):697. doi:10.3390/toxins15120697





















