Managing weight is one of the most powerful steps people can take to prevent or control type 2 diabetes. The American Diabetes Association’s 2024 Standards of Care note that even modest weight loss can improve blood sugar levels, while larger reductions may lower A1C, stabilize fasting glucose, and, in some cases, even lead to diabetes remission.
As more people search for effective and sustainable solutions, newer medications are stepping into the spotlight. One of the most talked about is Wegovy (semaglutide 2.4 mg) — a once-weekly injection designed for long-term weight management in adults and adolescents with obesity, and in adults with overweight who also have a related health condition.
Wegovy works by mimicking a natural hormone called GLP-1, which plays a key role in appetite and digestion. This helps reduce hunger and supports meaningful weight loss. In the sections that follow, we’ll look more closely at how it works, how it’s administered, and what patients and providers should consider before starting treatment.
Key Takeaways
- Wegovy (semaglutide 2.4 mg once weekly) has approval for chronic weight management in adults and adolescents (≥12 years) with obesity, in adults with overweight plus a related health condition, and to reduce cardiovascular risk in adults with established CVD and overweight/obesity.
- It functions as a GLP-1 receptor agonist, reducing appetite, delaying gastric emptying (most pronounced early in treatment), and promoting better metabolic control.
- STEP clinical trials demonstrated an average weight loss of ~15% in adults without diabetes, and smaller, yet still meaningful, results in those with type 2 diabetes. The SELECT trial demonstrated a 20% reduction in major cardiovascular events.
- High-dose semaglutide (7.2 mg) achieved ~21% weight loss in investigational studies, but this dose does not have FDA approval.
- Dosing begins at 0.25 mg and increases every 4 weeks, with a maintenance dose of 2.4 mg (or 1.7 mg if patients cannot tolerate 2.4 mg). In adolescents, the maintenance dose is 2.4 mg.
- Key safety considerations include risks of pancreatitis, gallbladder disease, hypoglycemia (with insulin or sulfonylureas), dehydration-related kidney injury, and diabetic retinopathy in T2D. It is contraindicated in patients with MEN2 or medullary thyroid carcinoma.
- For optimal results, experts combine Wegovy with a balanced diet, regular physical activity, and behavioral support as part of a comprehensive long-term weight management plan.
About: Medical Spa RX provides medical practices with premium products at the best prices. If you’re looking to buy Wegovy online for your practice, the sales representatives at Medical Spa RX can give you guidance.
Wegovy’s Pharmacodynamics: Semaglutide and GLP-1 Receptor Agonism
Wegovy (semaglutide 2.4 mg once weekly) is approved for chronic weight management in adults and adolescents (≥12 years) with obesity, in adults with overweight plus a related health condition, and to reduce the risk of major cardiovascular events in adults with overweight/obesity and established CVD.
Wegovy works as a GLP-1 receptor agonist, mimicking the body’s natural incretin hormone GLP-1. By binding to receptors in the pancreas and brain, semaglutide enhances glucose-dependent insulin release, reduces glucagon, and influences appetite control. Its long half-life allows once-weekly dosing with sustained receptor activation.
The gastric-emptying effect is strongest early in treatment and attenuates over time, leaving central appetite regulation as the primary long-term mechanism driving weight reduction. Collectively, these properties make Wegovy an effective tool for chronic weight management, while also improving glycemic measures in people with type 2 diabetes, even though it is technically not a treatment for diabetes.
How Wegovy Modulates Appetite, Gastric Emptying & Metabolic Pathways
The STEP clinical trial program successfully validated the mechanism of action behind semaglutide. Its effects span several domains of metabolism:

- Appetite Regulation: Wegovy acts on hypothalamic GLP-1 receptors to reduce hunger signals, increasing satiety and lowering calorie intake.
- Gastric Emptying: It slows how quickly food leaves the stomach, prolonging fullness and reducing post-meal glucose spikes. This effect lessens with continued therapy. Patients should inform anesthesia providers of GLP-1 use prior to procedures, as delayed gastric emptying can increase aspiration risk.
- Metabolic Balance: Semaglutide supports glucose-dependent insulin release and lowers hepatic glucose output by suppressing glucagon. These effects improve glycemic measures, particularly in those with type 2 diabetes.
Together, these pathways help explain the clinically meaningful weight loss achieved with Wegovy, as well as its impact on cardiometabolic health.
Wegovy in Clinical Trials: STEP & Other Semaglutide Programs
The STEP program established Wegovy’s efficacy across different populations:
- STEP 1–4: Adults with obesity and overweight showed substantial benefits, with STEP 1 reporting a mean of ~14.9% weight reduction over 68 weeks versus placebo in adults without diabetes.
- STEP 2: Focused on adults with type 2 diabetes receiving the approved 2.4 mg dose, showing clinically meaningful weight loss, though more modest than in non-diabetic populations.
- High-dose studies (investigational): Trials of 7.2 mg semaglutide (not approved) demonstrated a mean weight loss of ~21% over 72 weeks, with about one-third of participants losing at least 25% of their body weight. These findings highlight dose-responsiveness but are investigational and not part of approved therapy.
Beyond weight outcomes, the SELECT trial confirmed that Wegovy reduced major adverse cardiovascular events by 20% in adults with established cardiovascular disease and overweight/obesity. This adds an essential dimension to its clinical relevance.
Practical Considerations: Wegovy Dosing, Administration & Patient Counseling
Experts give Wegovy as a subcutaneous injection once weekly in the abdomen, thigh, or upper arm. Practical points for safe use include:
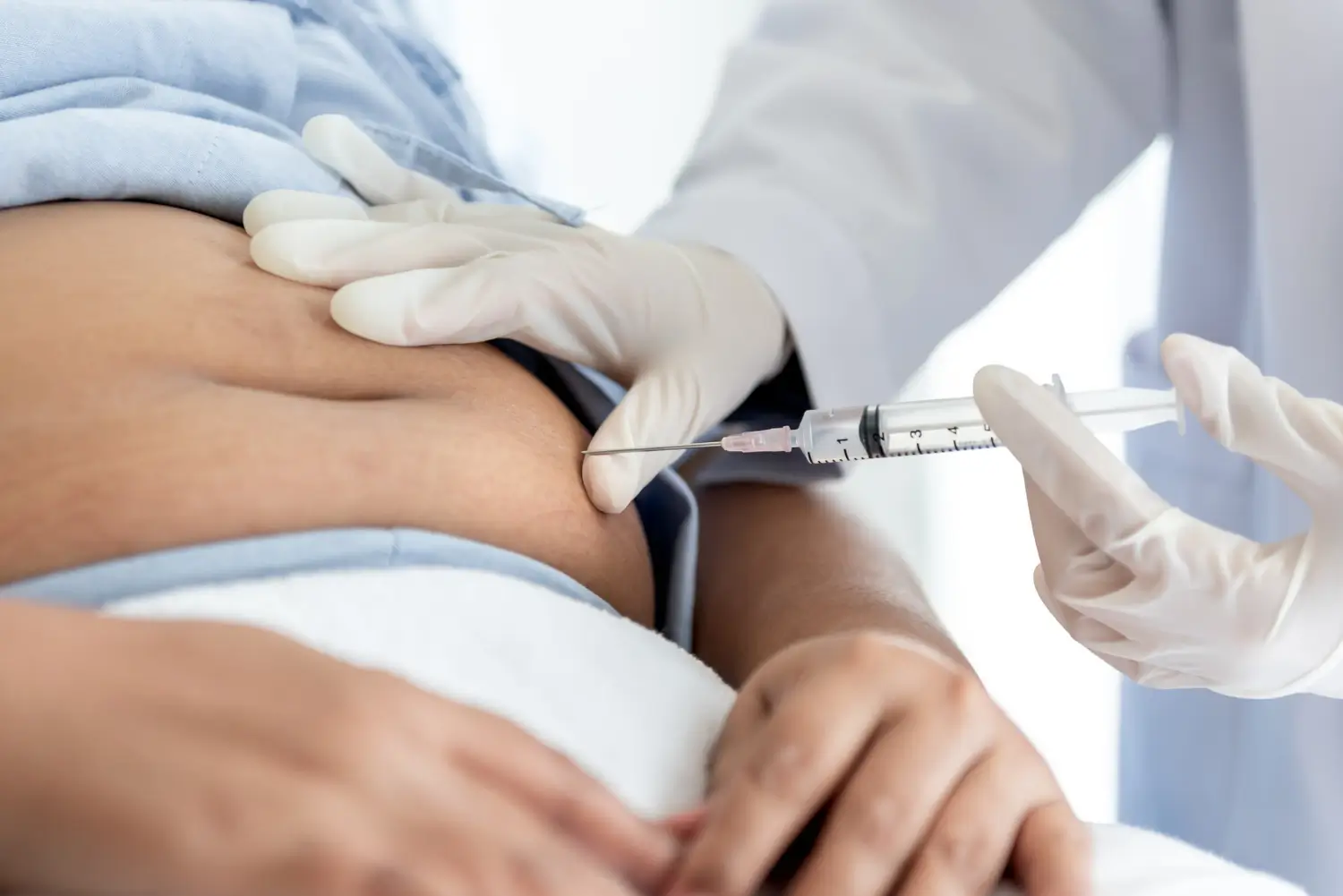
- Dosing and Titration: Start at 0.25 mg weekly, increasing every 4 weeks to a target of 2.4 mg. Adult maintenance may remain at 1.7 mg if they are unable to tolerate 2.4 mg. In adolescents, the maintenance dose is 2.4 mg.
- Missed Doses: If one dose is missed and the next is more than 48 hours away, administer the missed dose as soon as possible. If you miss ≥2 consecutive doses, resume as scheduled or consider restarting at a lower dose to minimize GI side effects.
- Contraindications: Wegovy should not be used in patients with hypersensitivity to semaglutide or its excipients, or in those with a personal/family history of medullary thyroid carcinoma or MEN2.
- Injection Technique: Rotate injection sites weekly. When administering, place the pen flat against the skin, unlock, and hold until the second click confirms delivery.
- Patient Support: Manufacturer programs or clinic-based coaching tools can aid adherence, lifestyle tracking, and behavior change.
Warnings and Precautions
Healthcare practitioners should monitor their patients for acute pancreatitis, gallbladder disease, worsening diabetic retinopathy in people with T2D, dehydration-related kidney injury, and hypoglycemia when combined with insulin or sulfonylureas.
Importantly, Wegovy is not recommended during pregnancy and should be discontinued at least 2 months before a planned conception. Conversations about Wegovy during pregnancy are critical in counseling women of childbearing potential.
Conclusion
Wegovy’s impact comes from its ability to regulate appetite, slow gastric emptying, and improve metabolic control, driven by semaglutide’s GLP-1 receptor agonism. Its efficacy has been confirmed in multiple STEP trials, with average weight loss approaching 15% in many patients and proven cardiovascular benefits in those with established CVD.
Practical use requires careful dosing, counseling, and monitoring, along with patient commitment to lifestyle changes. While higher investigational doses show further potential, the approved 2.4 mg regimen remains the standard. When prescribed appropriately, Wegovy provides a robust and evidence-based pathway for long-term weight management.
FAQs
1. What is the role of the GLP-1 receptor agonist mechanism in Wegovy?
It has FDA approval for chronic weight management in adults and adolescents (≥12 years) with obesity, and adults with overweight plus one weight-related condition. Experts also prescribe it to reduce major cardiovascular events in adults with overweight/obesity and established CVD.
2. How does Wegovy suppress appetite and affect digestion?
It mimics the GLP-1 hormone to reduce appetite, delay gastric emptying (effect strongest early), and improve glucose regulation.
3. What did clinical trials show?
STEP 1 showed ~15% mean weight loss over 68 weeks; STEP 2 confirmed efficacy in type 2 diabetes populations. SELECT demonstrated a 20% reduction in major cardiovascular events.
4. What about higher doses?
Investigational trials of 7.2 mg semaglutide showed ~21% weight loss at 72 weeks. However, this dose does not have FDA approval.
5. What side effects should patients expect?
Nausea, vomiting, diarrhea, constipation, abdominal pain, and fatigue are common early on and often lessen with continued use.
7. Is Wegovy a replacement for lifestyle changes?
No. It is most effective when combined with healthy eating, regular activity, and behavioral support.
References
ElSayed NA, Aleppo G, Bannuru RR, et al. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes–2024. Diabetes Care. 2023;47(Supplement_1):S145-S157. doi:10.2337/dc24-s008
Semaglutide for cardiovascular risk reduction in people who are overweight or have obesity without diabetes (new indication). Aust Prescr. 2025;48(3):107-108. doi:10.18773/austprescr.2025.024
Wegovy Dosage Guide. Drugs.com. https://www.drugs.com/dosage/wegovy.html
Wegovy® (semaglutide) injection 2.4 mg. novoMEDLINK. https://www.novomedlink.com/obesity/products/treatments/wegovy/about-wegovy/how-wegovy-works.html