EMLA™ 5x5g Indications for Use
EMLA® is a topical anesthetic cream used to numb the skin for various minor surgical procedures, such as blood sampling, vaccinations, insertion of intravenous catheters, and cleaning of leg ulcers. It is composed of a mixture of two local anesthetics: lignocaine (25 mg/g) and prilocaine (25 mg/g). The cream works by penetrating the skin, blocking the pain signals from reaching the nerves. This allows medical procedures to be carried out with minimal or no pain.
EMLA™ 5x5g Dosage Information
EMLA® is available as both a cream and a patch. The recommended dosage depends on the procedure and the area being treated. For most procedures:
- Apply at least one hour before the procedure.
- For male genital skin procedures, the cream should be applied for 15 minutes.
- For skin grafting, the cream should be applied for two hours.
- For leg ulcers, apply 30 minutes before cleaning.
Maximum doses for adults and children are provided in the package insert, and patients are advised not to exceed the recommended dosage.
EMLA™ 5x5g Side Effects and Precautions
Patients using EMLA® may experience certain side effects, which are generally mild and resolve quickly. These include:
- Local reactions: Itching, swelling, paleness, redness, or a burning sensation at the application site.
- More serious side effects: Rash, difficulty breathing, dizziness, blurry vision, or blue-tinted skin. These symptoms indicate a more severe allergic reaction and require immediate medical attention.
EMLA® should not be used on open wounds (except leg ulcers), and it is contraindicated for use in premature babies. In addition, patients with methaemoglobinaemia, dermatitis, or glucose-6-phosphate dehydrogenase deficiency should consult a doctor before using this product. Pregnant or breastfeeding women should use the cream only after consulting with their healthcare provider.
EMLA™ 5x5g Clinical Studies and Real-World Outcomes
EMLA® has been widely used and studied for its effectiveness in reducing pain for minor medical procedures. Clinical studies have shown that EMLA® significantly reduces pain when applied properly before procedures like vaccinations, venipuncture, and skin grafting. Results from real-world use indicate that EMLA® is particularly helpful for patients with a low pain threshold and for use in children undergoing painful procedures like blood sampling or IV insertion.
Drug Interactions
Patients using EMLA® should be cautious of potential drug interactions. EMLA® may interact with other local anesthetics, sulfonamide antibiotics, and antiarrhythmic medications like amiodarone. Patients are advised to inform their healthcare providers about any other medications they are taking to avoid complications.
EMLA™ 5x5g Administration and Aftercare
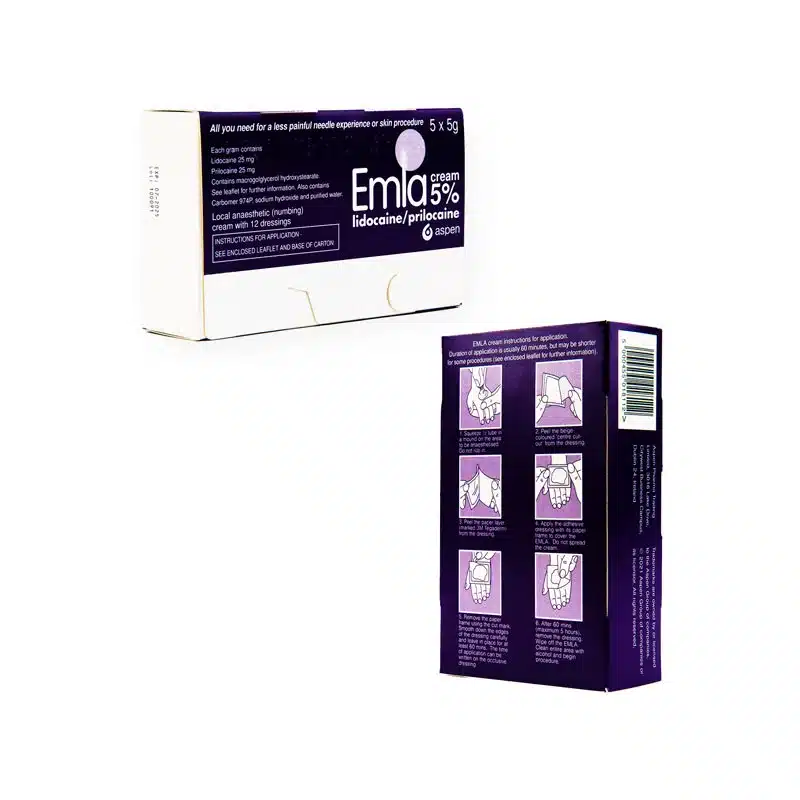
To administer EMLA® effectively:
- Apply the cream or patch at least one hour before the procedure. For some procedures like skin grafting, the application time may be longer (two hours).
- EMLA® should be applied to intact skin and covered with a dressing to ensure proper absorption.
- For leg ulcers, only a single-use tube should be applied, and any remaining cream should be discarded after use. The cream should not be applied on leg ulcers for longer than two months without consulting a healthcare provider.
EMLA™ 5x5g Storage and Disposal
EMLA® should be stored at temperatures below 30°C for the cream and 25°C for the patch. It should not be frozen. Patients should ensure that the cream or patches are kept in their original packaging until use to maintain effectiveness. To prevent accidental ingestion or misuse, EMLA® should be kept out of reach of children.
Unused EMLA® products should be disposed of properly, as advised by a pharmacist, especially if the product has expired or is no longer needed.