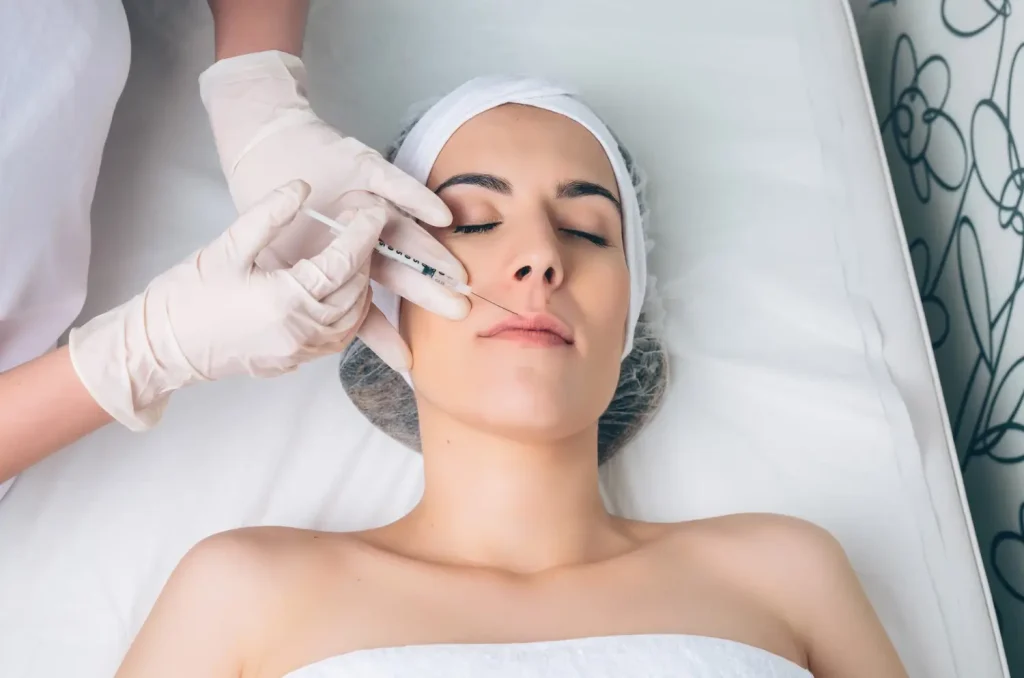
Fat-dissolving injectables first made their mark in late 1980s Europe, when clinicians began exploring the cosmetic potential of phosphatidylcholine to target localized fat deposits. In a groundbreaking self-experiment in 1995, dermatologist Patricia Rittes demonstrated that injections of phosphatidylcholine could effectively reduce subcutaneous fat, paving the way for formal clinical validation in the early 2000s.
Building on this legacy, Aqualyx utilizes modified bile salts, particularly deoxycholic acid derivatives, to disrupt fat cell membranes (adipocytes) and facilitate gradual fat clearance from targeted areas. Although Aqualyx is widely popular in Europe and carries a CE mark, which permits its cosmetic use, patients and practitioners in the United States are curious about whether it has approval from the U.S. Food and Drug Administration (FDA).
In this article, we’ll closely examine Aqualyx’s FDA approval status, explain its mechanism of action, and address the clinical, safety, and regulatory considerations healthcare providers and patients must navigate when choosing fat-dissolving therapies.
Key Takeaways
- Aqualyx is CE-mark certified in the European Union, widely used internationally for non-surgical fat reduction, but it does not hold FDA approval in the United States.
- Without FDA approval, Aqualyx is considered an unregulated injectable in the U.S., making its importation, distribution, and administration illegal under federal law.
- The FDA has explicitly warned against using unapproved lipolytics such as Aqualyx, citing serious health risks including infection, scarring, tissue necrosis, and disfigurement.
- Medical professionals in the U.S. risk severe legal, financial, and professional consequences, including lawsuits, disciplinary actions, license revocation, and loss of insurance coverage by using or offering Aqualyx treatments.
- Clinicians internationally customize the Aqualyx dosage according to the targeted area and patient needs, but such practices remain prohibited within U.S. borders.
- Several safe and FDA-approved alternatives exist for fat reduction in the U.S., including Kybella, CoolSculpting, and SculpSure, each thoroughly vetted for safety and efficacy.
- Patients seeking fat-dissolving treatments should always choose treatments performed by board-certified providers, prioritizing methods approved by the FDA to ensure safety, legality, and optimal results.
About: Medical Spa RX provides medical practices with premium products at the best prices. If you’re looking to buy Aqualyx online for your practice, the sales representatives at Medical Spa RX can give you guidance.
Aqualyx CE‑Mark and Global Usage
Aqualyx holds a CE-mark certification, indicating its compliance with stringent European Union health, safety, and regulatory standards. Widely embraced across countries such as the United Kingdom, Italy, Spain, and other European nations, Aqualyx has gained popularity due to its minimally invasive nature.
Patients also like its targeted effectiveness in reducing localized fat deposits. Common treatment areas include the chin, abdomen, thighs, and hips, attracting patients seeking non-surgical body contouring solutions.
Clinicians administer treatments by carefully adjusting the Aqualyx dosage to fit the targeted area, individual patient characteristics, and desired outcomes. Typically, treatments involve multiple sessions spaced weeks apart, allowing gradual fat clearance and refinement of body contours.
However, despite its acceptance across Europe and other global markets, the CE-mark does not equate to approval by the U.S. Food and Drug Administration (FDA). American patients and providers must recognize that international certifications do not translate to legal or safe usage within the United States, where regulations differ significantly.
Lack of FDA Approval for Aqualyx
Although Aqualyx enjoys extensive global popularity, it notably lacks approval by the FDA for use within the United States. This means:
- Aqualyx is not legally permitted for marketing, sale, or distribution in the U.S.
- Its importation, administration, or promotion by American medical providers violates federal regulations.
- FDA approval represents rigorous vetting for safety, efficacy, and manufacturing quality. Without this approval, Aqualyx remains an unregulated injectable, posing risks to both patients and providers.
Patients should be cautious of domestic practitioners offering treatments with Aqualyx, as regulatory authorities do not sanction such services and are likely to involve unauthorized or black-market sources. The absence of FDA oversight significantly amplifies health and safety risks associated with its use.
FDA Warnings on Unapproved Lipolytics
The FDA has explicitly issued public warnings regarding the dangers posed by using unapproved injectable lipolytic agents, including Aqualyx, based on adverse event reports. Documented risks and complications linked to unauthorized injectables include:

- Severe infections at injection sites, sometimes requiring hospitalization or surgery.
- Permanent scarring and significant disfigurement.
- Skin ulceration or tissue necrosis leading to irreversible damage.
- Inconsistent fat reduction causing contour irregularities, asymmetries, or unexpected deformities.
The FDA clearly emphasizes that unapproved injectables pose unknown risks, primarily due to a lack of controlled studies that verify their safety and efficacy. Furthermore, product sourcing from unregulated supply chains introduces uncertainties regarding purity, potency, and manufacturing practices. For optimal patient safety, the FDA advocates choosing only approved fat-reduction methods.
U.S. Legal and Professional Risks
Medical professionals in the United States offering or administering Aqualyx face severe professional, legal, and financial risks due to the absence of FDA approval:
- Increased potential for malpractice lawsuits arising from patient injury or unsatisfactory outcomes.
- Disciplinary action by state medical licensing boards, potentially resulting in suspension or revocation of medical licenses.
- Federal and state penalties, including fines, criminal charges, or imprisonment for illegally distributing unapproved drugs.
- Loss of coverage by professional liability insurance, as policies generally do not protect providers against claims arising from unapproved or unauthorized treatments.
Furthermore, claims of off-label use are invalid, as Aqualyx lacks any form of FDA recognition or investigational approval. Practitioners risk significant reputational damage and long-term consequences to their medical careers by using products outside the approved regulatory framework.
Safe and Legal Alternatives in the U.S.
Given the risks and limitations associated with unapproved fat-dissolving injectables, patients and providers in the United States have several safe, FDA-approved alternatives. These treatments meet rigorous regulatory standards for efficacy, safety, and manufacturing quality.
Kybella (deoxycholic acid)
- FDA-approved for submental (under-chin) fat
- Destroys fat cells permanently
- Administered in-office via injection
CoolSculpting (cryolipolysis)
- Non-invasive fat freezing treatment
- FDA-approved for multiple body areas
- No needles or downtime
SculpSure (laser lipolysis)
- FDA-approved laser-based treatment
- Heats and destroys fat cells
- Fast treatment sessions and minimal discomfort
These FDA-cleared alternatives provide patients with safe, reliable, and regulated options for body contouring, eliminating uncertainties associated with unregulated treatments like Aqualyx. Board-certified providers can guide patients toward the best method, tailored to individual goals, anatomy, and health status.
Conclusion
Despite its popularity in Europe and CE-mark certification, Aqualyx lacks FDA approval and remains illegal for use within the United States. The regulatory gap represents significant safety and legal risks for patients and practitioners. Without FDA oversight, Aqualyx’s quality, safety, and effectiveness cannot be guaranteed, exposing users to severe medical and legal consequences.
Fortunately, American patients have access to thoroughly vetted, FDA-approved fat reduction treatments such as Kybella, CoolSculpting, and SculpSure. These options have demonstrated safety, efficacy, and regulatory compliance, making them the responsible choice for cosmetic and clinical providers.
For safe, effective, and compliant care, patients should always consult licensed, board-certified professionals and avoid unapproved, unregulated, or black-market treatments.
FAQs
1. Is Aqualyx FDA-approved in the U.S.?
No. The FDA disapproves of Aqualyx and cannot be legally marketed or used in the U.S.
2. Can I legally receive Aqualyx in the U.S.?
No. Because Aqualyx is not FDA-approved, it is not legally available for administration or sale in the U.S.
3. Why isn’t Aqualyx approved in the U.S.?
The manufacturer has not submitted the necessary clinical data for FDA review and approval.
4. What are the risks of using unapproved fat dissolvers like Aqualyx?
Risks include infection, scarring, tissue damage, and complications from inconsistent product quality.
5. Are there safer alternatives to Aqualyx in the U.S.?
Yes. FDA-approved options, such as Kybella, CoolSculpting, and SculpSure, are available through licensed providers.
6. Can doctors offer Aqualyx off-label in the U.S.?
No. Aqualyx is not FDA-approved; therefore, off-label use does not apply.
7. Is Aqualyx safe if I get it done abroad?
Safety depends on the clinic, provider, and regional regulations. Always choose a licensed, reputable practitioner.
References
Hasengschwandtner F. Phosphatidylcholine treatment to induce lipolysis. J Cosmet Dermatol. 2005;4(4):308-313. doi:10.1111/j.1473-2165.2005.00211.x
Cohen P, Spiegelman BM. Cell biology of fat storage. Molecular Biology of the Cell. 2016;27(16):2523-2527. doi:10.1091/mbc.e15-10-0749
Nall R. About Aqualyx Fat-Dissolving Injections. Healthline. Published December 12, 2020. https://www.healthline.com/health/aqualyx